Deciphering protein complexes with Angstrom optical resolution
Cryogenic optical localization in three dimensions (COLD) has been shown to be a viable option for deciphering protein complexes at Angstrom-level. However, previous work was severely limited in the number of resolved fluorophores. A new approach, titled ‘polarCOLD’ has now pushed the boundaries for this research to new levels.
The recent advent of super-resolution (SR) fluorescence microscopy has witnessed many clever innovations and opened new avenues for studying sub-cellular organization and is on the way to become a workhorse for biological studies. However, conventional SR microscopy performed at room temperature is still not considered as a contestant in the arena of structural biology, where Angstrom-level information about the molecular architecture of single proteins and protein complexes is sought after.
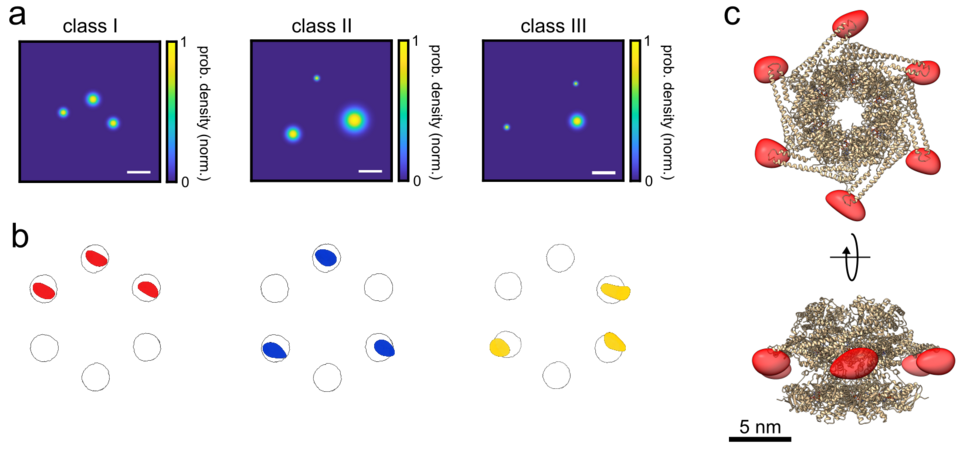
Cryogenic optical localization in three dimensions (COLD) was recently shown to resolve up to four binding sites on a single protein at Angstrom resolution. Because previous work with this relied on intensity fluctuations that result from the blinking behavior of fluorophores, it was limited to cases where individual emitters show different brightness, and thus severely limit the number of resolved fluorophores. A new paper, published in the journal eLife by Vahid Sandoghdar, Hisham Mazal and Franz-Ferdinand Wiesel from MPL has now advanced the frontiers of this research by extending the number of resolved fluorophores in a more robust manner and with higher yield.
Here, they combined polarization-encoded single-molecule localization at liquid helium temperature with partial labeling and 2D image classification schemes to reach Angstrom resolution in three dimensions. They demonstrated the performance of this method, which they termed ‘polarCOLD’, by resolving the distances and arrangements of different protein complexes, with three and six different sites. This new approach holds great promise for examining structure and different conformations of soluble and membrane protein complexes in challenging native environments. In addition, this method closes the gap between electron and optical microscopy and offers an ideal ground for correlating the two modalities at the single-particle level.
Photo: MPL
Contact:
Vahid Sandoghdar
vahid.sandoghdar@mpl.mpg.de
Publication:
Hisham Mazal, Franz Wieser and Vahid Sandoghdar, "Deciphering a hexameric protein complex with Angstrom optical resolution", https://elifesciences.org/articles/76308






